One of the most important aspects of a scientist's CV is the amount and size of grants or fellowships that we have obtained throughout our careers. However, the CV omits all the grant and fellowship applications that were not successful for any reason. I have decided to post here some of those research proposals that did not make it. Clearly, their panel of reviewers didn't know a thing ;)
This is my research proposal to an application I submitted to Institut Pasteur for the creation of new junior research groups. Enjoy!
The Photobiology Group
I propose here three multidisciplinary research
modules that will be the research core of the Photobiology Group:
1.
Functional
and structural characterization of
photochemical reaction centers
2.
Design,
remodeling, and enhancement of photosynthetic systems
3. Origin and evolution
of photosynthesis and bioenergetics systems
Photochemical reaction centers are distributed in
seven phyla of bacteria: Cyanobacteria, Chloroflexi, Firmicutes, Chlorobi,
Proteobacteria, Acidobacteria, and Gemmatimonadetes. Despite such great
biodiversity, today 95% of research in
photosynthesis is done in the Cyanobacteria/plant system, and less than 5% in
the remaining types of phototrophic systems (estimated by the total number of
publications in the last five years mentioning model organisms from each
group). From this 5%, 3.5% is research performed in a single strain of
Alphaproteobacteria, Rhodobacter
spheroides, probably best known for the 1988 Nobel Prize in Chemistry. We
know virtually nothing about most of the diversity of photosynthetic organisms
currently inhabiting Earth.
Even though the least studied
groups of phototrophs represent, at best, less than 1.5% of the total photosynthesis
research carried out in the world, there is a lot to be learnt from them. For
example, quantum coherence in living systems was first discovered in the FMO
light-harvesting complex of the Chlorobi1,
and potential applications of this quality may provide some insight to develop
more efficient solar cells2.
Strains of the phylum Chloroflexi have a unique carbon fixation pathway,
unlike those in plants and Cyanobacteria, which is thought to be more energy
efficient under specific conditions3.
Efforts to engineer this pathway in a model cyanobacterium have been attempted
recently4. Heliobacteria are
ubiquitous and powerful nitrogen fixers, commonly found in rice paddies around
the world5. They are some of
the fastest growing photoheterotrophic bacteria in nature; however, their
ecological importance has not been determined and their biotechnological
potential has not even been acknowledged in the literature. In conclusion, the
entire diversity of photosynthetic bacteria represents a new frontier of
research that if pursued, will certainly have a far-reaching societal and
technological impact.
Functional
and structural characterization of photochemical reaction centers
As mentioned above, reaction centers are distributed
in at least seven groups of distantly related bacteria. They come in two forms
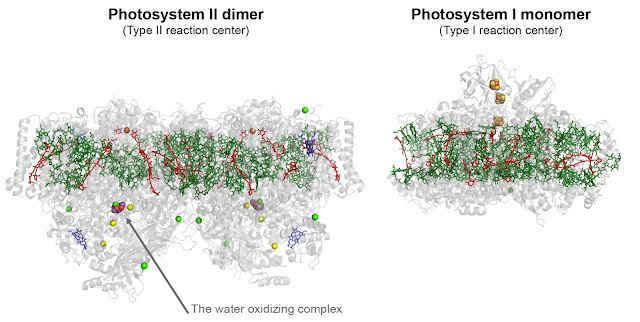
distinguished by the primary photochemical steps and known as Type I and Type
II reaction centers (Figure 1).
Today, there are crystal structures available for reaction centers in
Cyanobcateria, plants, and in Proteobacteria, but none in the other
phototrophic systems. The Photobiology Group will therefore obtain
high-resolution crystal structures of reaction centers from strains that are
very poorly understood; namely, Heliobacterium
modesticaldum (Heliobacteria), Roseiflexus
castenholzii (Chloroflexi), and Chlorobium tepidum (Chlorobi). Nonetheless, I already have in my
laboratory 8 additional strains selected because of their remarkable reaction
centers that I will bring with me to France.
I have selected those three
targets because their function and structure still remains to be elucidated.
Moreover, they contain immense evolutionary information. For example, the Type
I reaction center from Heliobacteria and the Chlorobi are the simplest in
nature and might be structurally similar to the earliest evolving systems.
However, their fundamental chemistry still remains a puzzle because the role of
quinones in electron transfer has not been conclusively demonstrated. It is
possible that under certain conditions these Type I reaction centers may behave
like Type II instead. Demonstrating that these simple reaction centers could
have a dual function would be a fantastic discovery that could change the way
we think about photosynthesis. On the other hand, the reaction center from Roseiflexus has a unique protein domain
not seen in any other proteins that could give clues on how water oxidation
catalysis evolved in Cyanobacteria6.
Experimentally the reaction
centers will be studied in vivo, in
isolated membranes, and in the purified enzyme. For example, excitation energy
and electron transfer under different conditions will be measured; as well as
any alterations to the energetics of cofactors, protein composition, or
oligomeric forms. Changes to the photosynthetic machinery under stress
conditions (e.g. high light intensity, iron or nitrogen starvation) will be
monitored too. The results will be compared to those in Cyanobacteria for which
extensive data is available. The key objective is to have a clear picture of
the function and dynamics of the reaction center in the target strains, a
picture that is not yet available to a satisfactory level of detail, if at all.
Simultaneously, crystal trials will be initiated as purified enzymes become
available. I have experience purifying the reaction center from H. modesticaldum, Photosystem II, and
Photosystem I. I also have experience with various spectroscopic methods such
as, absorption, fluorescence, and electron paramagnetic resonance (EPR)
spectroscopy; in addition to gel-based and gel-free proteomic approaches and in
the application of electrochemical methods to reaction centers. These
techniques will be applied judiciously in order to study function as deemed
necessary.
Currently, I am optimizing
crystallization conditions for the reaction center from H. modesticaldum; preliminary data suggests promising conditions.
These conditions and those available for Photosystem I7 could be used as a starting base for
the structure of Chlorobium. An
attempt at crystallizing the reaction center from Chloroflexus aurantiacus was published 20 years ago, but a
structure was never released8. This protocol could be further
improved for the structure of Roseiflexus.
Alternatively, modifications to available methods to crystallize Photosystem
II9 or the proteobacterial
reaction center10 could be
tried as well. Another possibility is to obtain structural models using
electron microscopy. The complete characterization and structural determination
of one or two of the reaction center shall make for a very exciting PhD project
or postdoctoral position, which should provide extensive results for multiple
high-impact publications.
Design,
remodeling, and enhancement of photosynthetic systems
We require a new source of energy. Biofuels from
photosynthetic organisms have been considered to be part of the solution to the energy crisis, but one of the grand
challenges is that overall, the efficiency of photosynthesis is low11. This is because the solar to biomass
energy conversion efficiency is around 1% or less (in real life, not under
optimal laboratory conditions). In other words, the ratio of energy returned
in the biofuel relative to the energy invested to produce it is currently quite
unfavorable, even in the best case scenarios. As a result, it has been
hypothesized that the natural limits of photosynthesis could be enhanced or
overcome12, but no experimental
validation of such hypotheses has been provided yet. All of the approaches
proposed to improve photosynthesis in living system require genetic
engineering. For example, it has been suggested that a reaction center could be
engineered to absorb light in the far-red region beyond the photosynthetically
active radiation, and this could potentially double its photosynthetic
efficiency. Such approach requires: 1) the expression of new pigment synthesis
pathways in parallel to the native ones, 2) the expression of a new reaction
center from a distinct organism into the host strain, or 3) both 1) and 2) at
the same time. However, we still do not completely understand pigment synthesis
and reaction center biogenesis. Although great advances have been made in the
past decades, still some of the steps, enzymes in the pathway, and assembly
factors, have not been identified or are very poorly characterized.
I
propose here a novel strategy―not yet discussed in the literature―to get great
insight into how correctly engineer a photosynthetic system and consequently,
how to improve it. The first stage of this module is to engineer photosynthesis
in a heterotrophic bacterium; or in other words, to reverse engineer
photosynthesis from scratch. My group will transfer a photosynthetic gene
cluster from a phototrophic gammaproteobacterium to Escherichia coli, which is also a gammaproteobacterium.
Genetically, they should be somewhat alike. Similar approaches have been
attempted before to engineer N2-fixation in E. coli successfully13. To do this, a nitrogenase gene cluster
from Klebsiella oxytoca containing
about 20 genes was refactored and then transferred into E. coli. K. oxytoca is
also a gammaproteobacterium. The photosynthetic gene cluster varies in size from
organism to organism ranging from 15 to 25 genes. Therefore, this technology
could be used as a starting foundation. We will take it several steps further.
In addition, photosynthetic
gammaproteobacteria are known to transfer genes in nature: for example,
Cyanobacteria of the marine Synechococcus
and Prochlorococcus clade have
obtained numerous photosynthetic genes from Gammaproteobacteria, including
circadian clock components, carboxysome components, Rubisco, chlorophyll
synthesis genes, among many others. Gammaproteobacteria have also been shown to
donate a photosynthetic gene cluster to strains of the rare phylum
Gemmatimondetes, and these have been demonstrated to be able to express
functional reaction centers14.
Horizontal gene transfer (HGT) events between organisms of different phylum
should be much more difficult to occur than within more closely related
bacteria, therefore I think inserting a photosynthesis gene cluster into E. coli is quite feasible. Furthermore,
several phototrophic gammaproteobacterial genomes are publicly available and
some strains are amenable to cultivation and genetic engineering. I will start
with the photosynthesis gene cluster of Thiocapsa
roseopercisina; an anoxygenic photosynthetic gammaproteobacterium, which
has been of particular interests because of its O2-tolerant
hydrogenase.
There are other alternatives that
can be tried in parallel. For example, the photosynthesis gene cluster of a heliobacaterium
(a Firmicutes) could be transferred to a strain of clostridia, their closest
non-phototrophic relative. Clostridia are a type of bacteria with immense
potential in the production of chemicals (e.g. ethanol, butanol, acetone, etc.)
and making them phototrophic may give current strains a technological and
renewable edge.
If a functional
reaction center can be engineered as a proof-of-concept in a non-phototrophic
bacterium, the possibilities to follow this up are limitless. First, selected
genes in the cluster could be removed or new ones added, in order to find the
minimum necessary genetic requirements for phototrophy and photoautotrophy. A
mix of genes from different organisms could be put together into novel gene
clusters using high throughput methods for generating combinatorial libraries
(e.g. through Golden Gate cloning) and for screening. Then it will be possible
to test whether functionality or activity yields could be improved or not. In
addition, the gene cluster could be inserted into strains of E. coli that have already been
engineered to produce diverse biofuels or compounds of interest to test if
production is coupled or enhanced with light utilization. A step farther would
consist in expressing a photosynthetic gene cluster in a yeast model. However,
the ultimate goal is to transfer a photosynthetic gene cluster encoding the
capacity for oxygenic photosynthesis from Cyanobacteria. A gene cluster for
oxygenic photosynthesis does not exist in nature, so it would be 100%
artificially designed. In this case, the engineered strain would use water and
light as the main energy source and thus would be completely photoautrotrophic.
This project, though risky, will provide invaluable insight into the nature of
photosynthesis and teach us immeasurably on the creation of novel life forms.
Origin
and evolution of photosynthesis and bioenergetic systems
Another one of my personal scientific interests is
evolution. How photosynthesis originated and diversified remains one of the
greatest puzzles in the history of life. I have set myself the personal goal to
reconstruct the most detailed evolutionary scenario yet for the origin and
diversification of photosynthesis. I have made good progress towards this with some
of my publications in the past four years6,15,16.
Earlier this year, I published a major reassessment of the evolution of
reaction centers6. In
addition, I led and published an exhaustive phylogenetic study of the D1
protein of Photosystem II, which provided for the first time, a clear picture
of how the water oxidizing complex of oxygenic photosynthesis evolved and the
dramatic transitions Photosystem II underwent in its path to acquiring water
oxidation catalysis16. My work
demonstrated how the structural and functional data available for Photosystem
II can be used to gain evolutionary information at an unprecedented level of
detail, if integrated with powerful phylogenetic analysis. As structural and
functional information from the studied photochemical reaction centers become
available in my group, these will be used to create even more precise molecular
evolutionary models.
At Institut Pasteur I also plan to
extend these evolutionary studies to the evolution of several major cofactor
synthesis pathways relevant to photosynthesis: namely, the chlorophyll, heme,
quinone, and carotenoid biosynthesis pathways. Understanding the evolution and
extent of the current diversity of cofactor biosynthetic pathway could come in
handy when redesigning and refactoring the photosynthetic gene clusters. It
could inform us on what genes or strains could be most promising. This would be
harder to achieve if a good understanding of the diversity and evolution of
phototrophy is lacking.
References
1 Engel, G. S. et al. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature (2007) 446, 782-786.
2 Park, H. et al. Enhanced energy transport in genetically engineered excitonic networks. Nat Mater (2015) doi: 10.1038/nmat4448.
3 Zarzycki, J., Brecht, V., Muller, M. & Fuchs, G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. PNAS (2009) 106, 21317-21322.
4 Shih, P. M., Zarzycki, J., Niyogi, K. K. & Kerfeld, C. A. Introduction of a synthetic CO2-fixing photorespiratory bypass into a cyanobacterium. J Biol Chem (2014) 289, 9493-9500.
5 Asao, M. & Madigan, M. T. Taxonomy, phylogeny, and ecology of the heliobacteria. Photosynth res (2010) 104, 103-111.
6 Cardona, T. A fresh look at the evolution and diversification of photochemical reaction centers. Photosynth res (2015) 126, 111-134.
7 Jordan, P. et al. Three-dimensional structure of cyanobacterial Photosystem I at 2.5 Å resolution. Nature (2001) 411, 909-917.
8 Feick, R., Ertlmaier, A. & Ermler, U. Crystallization and X-ray analysis of the reaction center from the thermophilic green bacterium Chloroflexus aurantiacus. FEBS lett (1996) 396, 161-164.
9 Umena, Y., Kawakami, K., Shen, J. R. & Kamiya, N. Crystal structure of oxygen-evolving Photosystem II at a resolution of 1.9 Å. Nature (2011) 473, 55-60.
10 Xu, Q. et al. X-Ray structure determination of three mutants of the bacterial photosynthetic reaction centers from Rb. sphaeroides; altered proton transfer pathways. Structure (2004) 12, 703-715.
11 Cotton, C. A., Douglas J. S., De Causmaecker, S., Brinkert, K., Cardona T., et al. Photosynthetic constraints on fuel from microbes. Front Bioeng Biotechnol (2015) 3, 36, doi: 10.3389/fbioe.2015.00036.
12 Ort, D. R. et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. PNAS (2015) 112, 8529-8536.
13 Smanski, M. J. et al. Functional optimization of gene clusters by combinatorial design and assembly. Nat Biotechnol (2014) 32, 1241-U1104.
14 Zeng, Y. H., Feng, F. Y., Medova, H., Dean, J. & Koblizek, M. Functional Type 2 photosynthetic reaction centers found in the rare bacterial phylum Gemmatimonadetes. PNAS (2014) 111, 7795-7800.
15 Cardona, T., Sedoud, A., Cox, N. & Rutherford, A. W. Charge separation in Photosystem II: A comparative and evolutionary overview. BBA-Bioenergetics (2012) 1817, 26-43.
16 Cardona, T., Murray, J. W. & Rutherford, A. W. Origin and evolution of water oxidation before the last common ancestor of the cyanobacteria. Mol Biol Evol (2015) 32, 1310-1328.
1 Engel, G. S. et al. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature (2007) 446, 782-786.
2 Park, H. et al. Enhanced energy transport in genetically engineered excitonic networks. Nat Mater (2015) doi: 10.1038/nmat4448.
3 Zarzycki, J., Brecht, V., Muller, M. & Fuchs, G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. PNAS (2009) 106, 21317-21322.
4 Shih, P. M., Zarzycki, J., Niyogi, K. K. & Kerfeld, C. A. Introduction of a synthetic CO2-fixing photorespiratory bypass into a cyanobacterium. J Biol Chem (2014) 289, 9493-9500.
5 Asao, M. & Madigan, M. T. Taxonomy, phylogeny, and ecology of the heliobacteria. Photosynth res (2010) 104, 103-111.
6 Cardona, T. A fresh look at the evolution and diversification of photochemical reaction centers. Photosynth res (2015) 126, 111-134.
7 Jordan, P. et al. Three-dimensional structure of cyanobacterial Photosystem I at 2.5 Å resolution. Nature (2001) 411, 909-917.
8 Feick, R., Ertlmaier, A. & Ermler, U. Crystallization and X-ray analysis of the reaction center from the thermophilic green bacterium Chloroflexus aurantiacus. FEBS lett (1996) 396, 161-164.
9 Umena, Y., Kawakami, K., Shen, J. R. & Kamiya, N. Crystal structure of oxygen-evolving Photosystem II at a resolution of 1.9 Å. Nature (2011) 473, 55-60.
10 Xu, Q. et al. X-Ray structure determination of three mutants of the bacterial photosynthetic reaction centers from Rb. sphaeroides; altered proton transfer pathways. Structure (2004) 12, 703-715.
11 Cotton, C. A., Douglas J. S., De Causmaecker, S., Brinkert, K., Cardona T., et al. Photosynthetic constraints on fuel from microbes. Front Bioeng Biotechnol (2015) 3, 36, doi: 10.3389/fbioe.2015.00036.
12 Ort, D. R. et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. PNAS (2015) 112, 8529-8536.
13 Smanski, M. J. et al. Functional optimization of gene clusters by combinatorial design and assembly. Nat Biotechnol (2014) 32, 1241-U1104.
14 Zeng, Y. H., Feng, F. Y., Medova, H., Dean, J. & Koblizek, M. Functional Type 2 photosynthetic reaction centers found in the rare bacterial phylum Gemmatimonadetes. PNAS (2014) 111, 7795-7800.
15 Cardona, T., Sedoud, A., Cox, N. & Rutherford, A. W. Charge separation in Photosystem II: A comparative and evolutionary overview. BBA-Bioenergetics (2012) 1817, 26-43.
16 Cardona, T., Murray, J. W. & Rutherford, A. W. Origin and evolution of water oxidation before the last common ancestor of the cyanobacteria. Mol Biol Evol (2015) 32, 1310-1328.
No comments:
Post a Comment