I have been recently reading about the interesting photosynthetic reaction center found in Heliobacteria. I think one of the most striking things about it is the debate and discussions about whether the Heliobacterial reaction center (RC) binds a quinone or not. The most recent and better purification strategy showed that there was 1.6 menaquinones per RC. They suggest that this could be interpreted as 64% of the centers having both quinones bound, 32% with one quinone bound and one empty site, and 4% with no quinones at all. Whatever the case the most likely interpretation about the whole debate―that has been going on for several decades as a matter of fact, is that the menaquinones in the Type I RC from Heliobacteria are loosely bound... the question is why and what is the relevance of this feature.
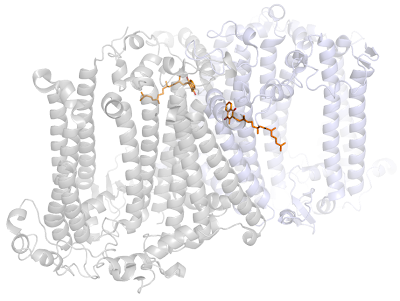
I wanted to see how the phylloquinones in Photosystem I are bound to the protein. You can see the figure below that most of the quinone is pretty much in a hollow space.
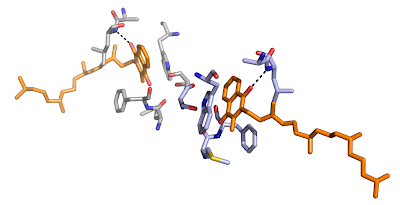
The head of the quinone interacts with the protein by a single hydrogen bond and by hydrophobic interactions to a tryptophan and phenylalanine side chain, see below.
Not only that, but the tail is surrounded by five chlorophylls and a carotenoid molecule, approaching within 4 Å of the quinone, see below.
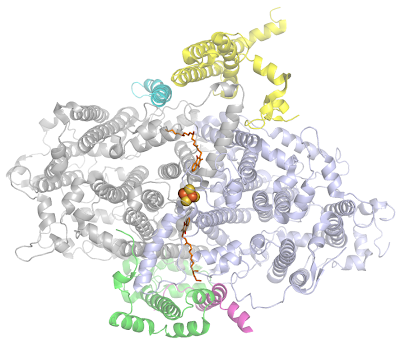
To top it all, these are held together by some of the smaller peripheral subunits:
So, no wonder why the quinones of PSI are so well bounnd.
In the case of the Heliobacterial reaction center, the tryptophan and phenylalanine are not conserved; there is only one carotenoid molecule (4,4'-diaponeurosporene) in comparison to 22 β-carotenes in Photosystem I; there are only about 20 chlorophylls in compared to about 96 in Photosystem I from Thermosynechoccocus; and there are no peripheral subunits. So, if the folding of the Heliobacterial reaction center protein is similar to Photosystem I, then the quinones will be very exposed.
I think a really interesting possibility is whether the Heliobacterial Type I could have a quinone reduction activity like in Type II reaction centers under certain conditions: this is the more likely when you consider that the PshB protein that should hold the terminal electron acceptors F(A) and F(B) is also loosely bound.